Honey is what bees make from nectar in order that it will keep; as such it contains what is present in the nectar minus some water. In fact it is much more complicated than that.
Honey is a complex mixture of:
- water
- sugar
- acids
- proteins
- enzymes
- vitamins and minerals
- enzymes.
1. Water
Approximately 17-20% of honey is water. One of the most important steps in the process is to reduce the water content to below about 20% because it has the effect of making honey indigestible to micro-organisms.
The amount of water the bees can remove depends on:
- the amount or water present in a nectar in the first place;
- the weather;
- the strength of the colony.
The amount of water in a nectar varies with the species of plant and with the weather. For instance – dandelion nectar has a higher sugar content than that of apple which produces a nectar ‘runny’ by comparison.
Superimposed on this is the weather effect. Many flowers tend to point their faces to the sun; this makes them very conspicuous to insects but if it rains then the flower fills with water and the nectar is diluted.
When ripening honey, bees fan at the entrance to pull air through the hive and over the honey to evaporate water from its surface. The warmer and drier the air, the faster the ripening. The amount of air passing over the honey would also speed evaporation and this last is related to the numbers of bees available for fanning. So if the weather is cold and wet and/or colony strength is down then the job is much more difficult and the honey may contain more water than is desirable.
So far, honey contains what was in the nectar and minus a quantity of water.
2. Sugars
Of the solids in honey, that is if all the water was to be removed, 95-99.9% is sugar of one sort or another, from the simple to the complex. To understand the complexity, and the simplicity, of sugars and the actions of enzymes it is useful to know a little about carbohydrate chemistry.
2.1. Carbohydrate chemistry
Simple sugars are called monosaccharides. There are very many different species of monosaccharides but what they all have in common is that they are built from a number of carbohydrate sub-units. The basic formula of a carbohydrate is CH2O or H-C-OH. Each molecule of carbohydrate may be thought of as one of the vertebrae which, linked together, make up the spine of a simple sugar and to which, other chemical appendages may, or may not be attached.
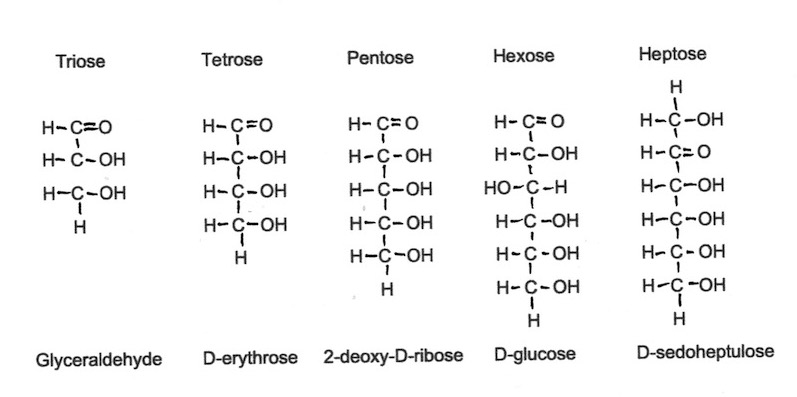
Monosaccharides can have a spine of three, four, five, six or seven individual carbohydrates and are known as triose, tetrose, pentose, hexose or heptose monosaccarides respectively. Glucose and fructose are hexose monosaccharides and both are very common in honey.
In Figure 1 below, each C represents a Carbon atom, H is a Hydrogen atom and O is an Oxygen atom. The black lines between them represent the bonds holding the individual atoms together as a molecule. It is important to understand that the atoms illustrated here are not welded permanently into place – it is more as if they are held together by forces similar to magnetism.
Figure 1. Carbohydrate skeletons of 4 monocaccharides.
There are two ways in which each of the above molecules can assemble so that two forms of each exist which are essentially the mirror image of each other – rather like a pair of gloves and they are known as L and D forms. D is for ‘dexter’ which is Latin for right, L is for ‘læve’, Latin for left – although these refer to the direction in which they rotate polarised light and not their handedness.
Sugar molecules, such as those above, in a solution such as in honey, tend to curl up into rings with an oxygen atom forming the clasp so to speak. There are two ways they can do this too, and they are known as a or bconfigurations (see fig 2 below). A solution of pure glucose will contain equal quantities of a & b.
a-D-glucose b-D-glucose
Figure 2 a and b-D-glucose.
There are many ways the different forms of the various monosaccharides can link up but a molecule made of two linked monosaccharides is always termed a disaccharide. Example of disaccharides would be
- sucrose which is made of a-D-glucose and b-D-fructose (see fig 3 below);
- maltose which is made up of two a-D-glucose molecules.
Figure 3. Disaccharide molecule of sucrose made up of a-D-glucose and b-D-fructose. Note carbon atoms are not marked but there is one at each otherwise un-labelled elbow or junction. Invertase is an enzyme which breaks the sucrose disaccharide into its composite monosaccharides but this diagram fails to show the inversion of D-fructose to L-fructose.
(From http://www.njsas.org/projects/light_polarization/sugars.html )
When there are three monosaccharides the resulting sugar is known as a trisaccharide, four is a tetrasaccharide. Sugars involving two, three or four simple sugars are known as oligosaccharides. More than that and they are known collectively as polysaccharides or higher sugars. The structures of these become more and more complex with size.
After all that, the important bits to note are:
- the enormous variety of sugars that can be made;
- the dynamic nature of the bonds between monosaccharides and between the atoms of which they are made;
- The relative ease with which they can be either pulled apart and reassembled, or agglomerated into something bigger;
As such they can quite easily be broken up when exposed to stronger forces, such as an enzyme (see below) or if physical (e.g. heat) or chemical conditions (e.g. acid) weaken the existing bonds. It is these fluid chemical properties that make honey the malleable dynamic product it is.
“All is flux. Nothing stays still, and nothing endures but change.” Heraclitus (540-480BC).
2.2. Sugars in honey
Many years ago, it was thought that honey was a mixture of dextrose and levulose, sucrose and a vague carbohydrate known as ‘honey dextrin’. Dextrose is actually D-glucose and Levulose is another name for L-fructose, both are breakdown products of sucrose (see figure 3.).
Today’s powerful analytical techniques have shown that in addition to those three, honey is a much more complex mixture of some formidably named sugars such as:- kojibiose, isomaltose, nigerose, ab trehalose, gentiobiose, laminaribiose, meleziotose, maltotriose, turanose, 1-kestose, panose, maltulose, isomaltotriose, erlose, theanderose, and O-a-D-glucopyranosyl-(1->6) -O-a-D-glucopyranosyl-D-fructose… the list goes on through about 25 or more in total (White 1979). It is thought that many of these sugars are not present in nectar but are formed by the dual actions of honey acids and enzymes during ripening and storage of honey. There is a good chance that at least some of them are ephemeral pre-cursors to, or by-products of, on-going reactions.
Despite this though, it seems that after all that, those characteristics that make honey honey are due to the relatively simple disaccharides – dextrose (35%) and levulose (40%) (Hooper). Generally there is more levulose than dextrose and only in those honeys that are quick to granulate is there more dextrose than levulose.
Sugars and water together make up about 97% of honey (Hooper), the remaining 3% is made up of the following ingredients.
3. Acids
There are a multitude of acids present in honey. If expressed as a percentage of honey, all the acids together contribute between 0.31% and 0.82% of volume and this creates a pH of about 3.2 – 4.5 (White 1975). pH is a measure of the free Hydrogen ions that acids contribute a solution, the smaller the number, the more acid a solution. The acids present in honey fall into 3 main categories: organic, inorganic and amino acids.
3.1. Organic acids
The organic acid portion is made up of about 15 organic acids including acetic, butyric, formic, gluconic, malic and succinic (Hooper, White 1975). The most abundant of these is gluconic acid (White 1979) which is a by-product of the breaking down of the glucose in dextrose. An enzyme, glucose oxidase catalyses glucose to form gluconic acid and hydrogen peroxide. The latter is the main agent responsible for the antibacterial activity in most honeys. The antibacterial properties of honey are well known and have caused it to be used as a successful wound dressing (Hooper).
3.2. Inorganic acids
Phosphoric and hydrochloric (Hooper, White 1975) and Sulphuric (White 1975) acid are inorganic acids present in honey.
3.3. Amino acids
A total of 17 of the 20 amino acids have been found in different honeys but the average in any one honey would be around 11 (White 1975) including proline, glutamic acid and lysine (Hooper). Apart from contributing to the pH of honey, amino acids are the building blocks of all proteins – including ourselves.
4. Proteins and Enzymes
4.1. Proteins
A protein is a polymer, or chain, of amino acids linked together rather like the sugars above. Molecular attractions between constituent amino acid sub-units cause the chain to fold up into a convoluted shape which is specific, and generally useful, to the protein in question. Proteins are denatured by heat, cold and pH which can all deactivate them. Fortunately, or unfortunately, proteins make up a portion of honey so small as to make a major sortie into their biochemistry unnecessary. Proteins in honey include albumins, globulins, proteoses and peptones and are of mixed bee and plant origin (White 1975). Enzymes are a dynamic, chemically active family of proteins (see below).
4.2. Enzymes
Enzymes are large, complex, disturbingly ‘clever’, chemically active molecules of protein. The names of enzymes always end in an ‘ase’, the front part giving some indication of action they carry out. Invertase, for example, splits sucrose molecules then inverts the free D-fructose into L-fructose.
Each molecule of an enzyme can be imagined as being like a tiny pair of hands which, in the presence of its specific target molecule, will locate on, or curl their ‘fingers’ around the specific target molecule in such a way as to pull it apart. All this takes place spontaneously and is brought about by the changing attractions between constituent molecules in the enzyme and in the target. However, there is something unearthly about the way the very action of bearing down on the target molecule causes shape changes in the structure of the enzyme, perhaps bringing molecules that were outside each other’s sphere of influence closer together until they are close enough to either attract or repel each other in such a way as to cause the enzyme to tear the spine of the target. In the case of invertase, it gives sucrose a good old twist into the bargain. Enzymes can be activated or deactivated by changes in temperature or pH or by concentrations of target and/or resultant molecules.
Enzymes in honey are of both bee and plant origin and include:
- Invertase which breaks sucrose (see figure 3) to D-glucose and D-fructose then twists the latter into L-fructose. Invertase is added by the bees (White 1975);
- Amylase (a and b) – a amylase splits a starch chain randomly into sugars but b amylase breaks maltose from the ends. Amylases are thought to originate from pollen (White 1975);
- Diastase is a name that is applied to both amylases when its presence in honey is to be assayed as a measure of the amount of heat a honey has been subjected to. It degrades with heat (White 1979) so the degree of degradation (loss of activity) can be used as a measure of the amount of heat that has been applied. Diastase also deteriorates with storage;
- Glucose oxidase originates in the pharyngeal glands of bees and oxidises glucose to gluconic acid, a by-product of this is hydrogen peroxide which is an antibiotic. Before this discovery, the observed antibiotic effect was at one time attributed to the action of something named inhibine.
- Catalase and phosphatase are also present in honey.
4.3. Colloids
Colloids are included among the proteins because it seems they are thought to be tiny fragments of plant material or large molecules including protein (Hooper); however, they are in suspension rather than in solution like proteins and enzymes, but are just too small to be removed by filtration.
According to White (1979) they are gummy, noncrystalline substances consisting of proteins, waxes, pentosans, and inorganic constituents. Chambers defines a colloid as being any soluble substance that does not pass through a membrane when exposed to dialysis – from the Greek kolla meaning glue and eidos meaning form. Hooper elucidates by saying that colloids repel each other having similar electrical charges and that this repulsion slows movement and therefore adds to the viscosity of a honey. Dark honeys contain about 5 times as much colloidal matter as light honeys.
5. Minerals
The mineral content of honey is determined by analysis of the ash that remains if honey baked at high temperatures. Ash content averages about 0.17% of its weight but varies between 0.02% and 1.0%.
5.1. Mineral elements
In this tiny portion about 12 mineral elements are present including potassium, sodium, calcium, magnesium, iron and copper although they are present in tiny quantities. In even tinier quantities are the trace elements.
5.2. Trace elements
These too are determined from their presence in ash. About 17 trace elements are present in even tinier quantities – hence the name. These include chromium, lithium, nickel, lead, strontium, silver and even gold! (White 1975).
6. Vitamins
The vitamin content of honey is very variable between samples but honey generally is thought to contain thiamin riboflavin (B2), pantothenic acid, thiamine, pyridoxine and ascorbic acid (C). The vitamins found in honey are thought to be of little dietary significance bearing in mind the average consumption of honey.
7. Hydroxymethylfurfuraldehyde (HMF).
This chemical is thought to be formed by the decomposition of fructose in the presence of acid although there is also a small amount of HMF in honey in hives. It is produced naturally as honey ages but the process is accelerated by heating so HMF can be used, along with diastase, as a measure of the amount of heat that honey has been exposed to.
8. Aroma and flavour
Also present in honey are grains of pollen and aromatic chemicals from the various flowers visited and these all contribute to the aroma and flavour of honey. In fact the delightful aroma and flavour of honey is probably the reason bees have had to evolve the sting so as to defend themselves against predators. Somewhere in the jungles of south America, or perhaps Africa, is a species of honey bee that gets by without the need for a sting; instead it has evolved the practice of visiting putrefying corpses to the extent that their honey takes on such a repulsive odour nobody wants it. Of course there is always the danger that hyenas or vultures will evolve a liking for it because evolution’s like that.
9. Toxicity
If bees have visited quantities of poisonous plants the honey may have an element toxic to either bees or to humans or to both. There is evidence that too much red chestnut honey is bad for bees. Honeys toxic to humans have been reported as coming from Rhododendron spp., Egyptian henbane (Datura metel) deadly nightshade (Atropa bella-donna) and Arbutus unedo from Sardinia. Arbutus unedo is also known as the strawberry tree which grows wild in the south west of Ireland. There are many poisonous plants in Ireland but it is unlikely that any of them grow in such profusion as to poison honey.
10. The rest
There are other substances present in honey and more will be discovered as analytical methods improve but until then we will have to make do with these.
Copyright © Beespoke.info 2022. All Rights Reserved.
References and Bibliography
Chambers Twentieth Century Dictionary. London. 1901.
Fitter,R., Fitter,R. & Blaney,M. The Wild Flowers of Britain and Northern Europe. Collins London. 1985.
Hooper,T. Guide to Bees and Honey. Blandford, London. 1991.
Kotz,J.C. & Purcell,K.F. Chemistry and Chemical Reactivity. Saunders College Publishing. USA. 1987.
Salisbury,F.B. & Ross,C.W. Plant Physiology. Fourth Edition. Wadsworth Publishing Company, Belmont, California. USA. 1992.
White.J.W. Honey. In The Hive and the Honey Bee. Ed. Dadant and Sons. Dadant Publications. Illinois. USA. 1979.
White.J.W. Composition of Honey. In Honey: A Comprehensive Survey. Ed. Eva Crane. Heinemann, London. 1975.
Links
Click this for more about enzymes in honey
Click here for honey, healing and hayfever
Click for how to take a crop of ivy honey
Click here for how to take a crop of heather honey
Click here for how to get section honey
Click here for the full index of posts
Copyright © Beespoke.info 2022. All Rights Reserved.