Oxalic acid is known to beekeepers because:
It affects Varroa directly and indirectly. Directly – the acid damages their mouthparts. Indirectly – it increases grooming activity between bees and more Varroa are dislodged in the process.
Also…
- The mites seem unable to acquire resistance to it;
- It doesn’t accumulate in beeswax;
- Doesn’t harm the bees;
- It is an organic remedy;
- And a harmless natural component of honey.
But there’s more…
A little bit of chemistry
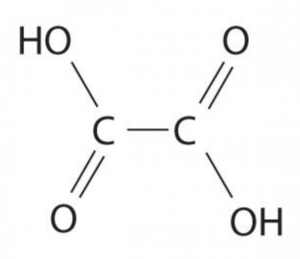
Oxalic acid is an organic acid also sometimes known as ethanedioic acid. The chemical formula is H2C2O4 which means there are 2 hydrogen, 2 carbon and 4 oxygen arranged as per this diagram:
The carbons form the spine of the molecule. They are each attached to the other and to an oxygen (O) and a hydroxide (OH or HO) group. It is usually sold as oxalic acid dihydrate in which case two additional water molecules are also involved.
Oxalic acid in nature
Oxalic acid is present naturally in many plants but most famously in the leaves of rhubarb (Rheum rhabarbarum), and wood sorrel (Oxalis acetosella) and common sorrel (Rumex acetosa). Rhubarb stalks are edible but we all know from our Mothers that the leaves are toxic. However, it seems we would need to eat around 5kg of leaves to get a lethal dose but it does interfere with calcium uptake so be careful all the same.
And as for wood sorrel, you will recognise it from spring woodlands. Next time you see it – pluck a leaf and eat it – lovely in salads but don’t eat too much. Here it is:
Common sorrel is edible too. It is a member of the dock family but the leaves are smaller and shaped like arrowheads. The flavour is acidic, green and intense – nice in salads and I bet it would make an interesting pesto. It looks like this:
It is also in honey!
Oxalic acid is commonly present in many other plants too so it should come as no surprise that it is a constituent of most honeys. Concentrations in honey vary depending on the botanical origin of the nectar of course – in the region of 3.3–761.4 mg/kg.
Values for honeys we are familiar with in this part of the world are as follows:
- Wildflower honey 8–51 mg/kg;
- Heather 48–151 mg/kg and 85.5–168 mg/kg;
- Oilseed rape honey 13–53 mg/kg;
- Generally honey contains < 200 mg oxalic acid/kg honey.
Toxicity to humans
- It is not a carcinogen;
- It is extremely corrosive and will cause burns to eyes, skin and respiratory tract;
- It may cause kidney damage;
- May interfere with calcium uptake;
- It has been reported that the lethal oral dose is 15 to 30 grams.
Click here for Oxalic Acid Safety Data sheet
Miticide nitty-gritty
Oxalic acid is only useful against Varroa when there is a broodless period in the honeybee colony and the mites are phoretic. ‘Phoresis’ is a distribution phase in the life of a parasite when it is out in the open and moving about on the host. Otherwise the Varroa mites are safely inside the sealed honeybee brood where the oxalic acid cannot reach them.
Broodless periods occur naturally thoughout the year:
- During mid-winter quiescence;
- In summer after a swarm there is a broodless period before the new queen starts to lay;
- After day 21 of an Artificial Swarm;
- If you use Snelgrove boards it can be incorporated into either of Methods 1 or 2. Click here for L.E.Snelgrove book review;
- Queenlessness.
Oxalic acid can be used effectively during any or all of these periods.
Although oxalic acid concentrations in honey do not increase significantly when bees are treated it is best to remove supers during treatment if treating during summer.
Oxalic acid can be applied in different ways:
- Vapourising or ‘sublimation’ method
- Trickle method
Vaporising / Sublimation method
For this, a Varrox vaporiser is required. Basically it is a little pan on a stick attached to a power source. Oxalic acid is placed in the pan and inserted through the hive entrance. The hive is then sealed, the power is turned on and the little pan heats up and the acid is vaporised in the hive. The bees seem unconcerned but the mites drop like flies. This is more suited to winter application.
Click here for more details on winter vaporising.
Trickle method
For the Trickle method, much research has been carried out to determine the concentration of oxalic acid that will kill most mites while causing least damage to the bees. The consensus, for this part of the world anyway, is 3.2%. Personally I have been using 3.3% for about 10 years with no observable ill-effects.
Click here for recipes for both concentrations and instructions.
Click here for Winter Oxalic Acid Treatment
Click here for Summer Oxalic Acid Treatment
Click here for more on Snelgrove
Click here for pictures and how to make your own Snelgrove board
Click here for Varroa Floor Flaw
Sources
Aliano, N. An Investigation of Techniques for using Oxalic Acid to reduce Varroa mite populations in Honey Bee Colonies and Package Bees. (2008) University of Nebraska
Nanetti, A., R. Büchler, J.D. Charrière, I. Fries, S. Helland, A. Imdorf, S. Korpela, and P. Kristiansen. Oxalic acid treatments forVarroa control (Review). (2003) Apiacta 38: 81-87
Rademacher, E.R & Harz, M. Oxalic acid for the control of Varroosis in honey bee colonies – a review. (2006) Apidologie 37: 98–12
Rashid, M, Wagchoure, E.S., Mohsin, A.U., Raja, S., Sarwar, G. Control of Ectoparasitic Mite Varroa destructor in Honey Bee (Apis mellifera. L.) colonies by using different concentrations of Oxalic acid. (2012) Journal of Animal and Plant Sciences, 22(1): 72-76
Copyright © Beespoke.info, 2014. All Rights Reserved.